2123rd Stated Meeting | March 26, 2024 | Henry R. Luce Hall, Yale University
On March 26, 2024, stem cell biologist Haifan Lin received the Francis Amory Prize of the American Academy of Arts and Sciences. First awarded in 1940, the Amory Prize recognizes significant scientific advances in reproductive biology and medical care. The award ceremony included remarks by Yale University President Peter Salovey and Academy President David W. Oxtoby, a reading of the Amory Prize citation by Dean of the Yale School of Medicine Nancy J. Brown, and a presentation by Professor Lin. An edited version of the remarks and presentation follows.
Peter Salovey
Peter Salovey is President of Yale University and the Chris Argyris Professor of Psychology. He was elected to the American Academy in 2013.
Good afternoon and welcome. It gives me great pleasure to call this meeting to order. This auditorium is filled with Professor Haifan Lin’s colleagues and friends from the School of Medicine and from across the university, as well as from the American Academy of Arts and Sciences. Thank you to those of you who came from out of town to be here with us today.
It is wonderful to welcome the American Academy to Yale’s campus for this occasion, especially David Oxtoby, president of the Academy, whom we will be hearing from momentarily. I want to thank the Academy for bestowing this award on our colleague. At Yale, we are animated by our mission to improve the world for this and future generations through outstanding research, scholarship, and education. And there can be no doubt that Professor Lin contributes to that mission every day. Over his career, Professor Lin has made seminal advancements in the fields of reproductive biology, developmental biology, and stem cell biology. His achievements range from the demonstration of stem cell asymmetric division, to the proof of the stem cell niche theory, to the discovery of the Argonaute/Piwi protein family and examination of its function in stem cell maintenance, germline development, epigenetic programming, and post-transcriptional regulation.
Professor Lin and others have also discovered millions of small noncoding RNAs that are encoded by junk DNA and partner with Piwi proteins called Piwi-interacting RNA—a discovery hailed by Science magazine as one of the top ten breakthroughs of 2006. Professor Lin has done groundbreaking research on stem cells and their use in the diagnosis and treatment of disease. And the potential applied implications of that work are quite dramatic.
As Yale’s president, I would like to convey my gratification and appreciation that under Professor Lin’s leadership, the Yale Stem Cell Center has grown into a vast network involving many faculty members from across the university, and spanning several departments and disciplines. What began as a modest undertaking comprised of two founding labs now has over one hundred participating labs. It is one of the largest stem cell research organizations in the world. I was in Hong Kong last week and the president of the Chinese University of Hong Kong was telling me about Professor Lin’s Stem Cell Center.
Guided by Professor Lin’s curiosity, quest for knowledge, and enthusiasm, the Center aims to advance our understanding of human stem cell biology and to harness its potential to improve health by removing the barriers between the present and the possible.
Professor Lin, all of us at Yale take immense pride in this recognition of your contributions to research, education, and indeed to humanity. Yale University’s success in such a vital field of scientific inquiry would not have been possible without your brilliance and energy. I want to thank you for the distinction you bring to this university and for your contributions to the world.
David W. Oxtoby
David W. Oxtoby completed his term as President of the American Academy of Arts and Sciences in June 2024. He was elected a member of the American Academy in 2012.
Thank you, Peter. We are grateful for your hospitality and so glad to be here to celebrate Haifan Lin and to present him with the Academy’s Amory Prize for reproductive biology. Congratulations, Peter, on an incredible decade as president of Yale. As I’m also nearing the end of my presidency, I want to take the opportunity to thank you for your partnership both as an individual Academy member and as the leader of an essential institution in our Affiliate network. As an Affiliate, Yale provides crucial support for the work of the Academy.
I also want to thank the cochairs of the Academy’s New Haven Program Committee—Isabela Mares, professor of political science, and Steven Wilkinson, director of the MacMillan Center—for their work to foster community among Academy members. We are proud to have over 180 Academy members affiliated with Yale University.
Yale members are deeply involved in the work of the Academy, from convening important conversations on topics like the global order to contributing to Academy projects. As a recent example, political scientist Jacob Hacker has been part of our Commission on Reimagining Our Economy (CORE) and was instrumental in designing the CORE Score: a new people-centered indicator of American well-being. Yale members also support one another. We are grateful that so many came here to celebrate a friend and colleague this evening.
In keeping with an Academy tradition that dates back to our founding in 1780, it is now my pleasure as president to officially open the 2123rd Stated Meeting of the American Academy of Arts and Sciences.
The American Academy was founded by a group of scholar-patriots to guide the new nation with a mission to “cultivate every art and science which may tend to advance the interest, honor, dignity, and happiness of a free, independent, and virtuous people.” For over 240 years the Academy has celebrated excellence in all forms of inquiry by electing members who represent the very best of their fields.
We also celebrate excellence through eleven prizes that are awarded in recognition of remarkable contributions to science, the humanities, and the ideals of the Academy. This year, under the leadership of Prize Committee Chair Pauline Yu, president emerita of the American Council of Learned Societies, the Academy will confer just two prizes: the Don M. Randel Award for Humanistic Studies to Kwame Anthony Appiah, and the Francis Amory Prize to Haifan Lin.
The Francis Amory Prize was first awarded In 1940 to recognize outstanding scientific achievements in the field of reproductive biology. The history of the Amory Prize chronicles the trajectory of the field, which has advanced significantly over the past eighty-four years as scholars continue to build on the discoveries of their colleagues who came before. Modern recipients of this prize include David Page in 1997, Patrick Walsh in 2012, Barbara Jean Meyer in 2017, and, most recently, Ruth Lehmann and Gertrud Schüpbach, who were awarded jointly in 2020 for advancements in DNA repair, embryonic development, RNA regulation, and stem cell research.
Tonight we celebrate Haifan Lin for his numerous and essential discoveries in stem cell research. For many of you, Haifan may be a mentor, a source of inspiration, a colleague, or friend. We are proud to call him a member of the American Academy; he was elected in 2018.
It is now my pleasure to invite fellow Academy member Nancy Brown, the Jean and David W. Wallace Dean of Medicine and the C.N.H. Long Professor of Internal Medicine at Yale School of Medicine, to shed further light on why Haifan is so deserving of this honor.
Nancy J. Brown
Nancy J. Brown is the Jean and David W. Wallace Dean of Medicine and the C.N.H. Long Professor of Internal Medicine at Yale School of Medicine. She was elected to the American Academy in 2021.
Before I read the award citation, let me mention Haifan’s many titles and the departments that he is affiliated with. He is the Eugene Higgins Professor of Cell Biology; Professor of Genetics, of Obstetrics, Gynecology & Reproductive Sciences, and of Dermatology at Yale School of Medicine; and Director of the Yale Stem Cell Center. All of that speaks to the fact that, Haifan, you are a leader among other thought leaders, not just in the School of Medicine, but internationally. You are an incredible mentor and many of your mentees as well as your peers are here today.
I am always grateful for your advice in the School of Medicine. You were recruited to lead the Yale Stem Cell Center in 2006 by Bob Alpern, who is in the audience today. And as President Salovey mentioned, under your leadership the Center has grown to over one hundred faculty and it is continuing to grow.
It is now my pleasure to read the prize citation.
Established in 1940, the Francis Amory Prize is awarded to an individual for their overall contributions to and influence in the area of reproductive medicine and physiology.
For his distinguished achievements, the American Academy confers the Francis Amory Prize on Haifan Lin.
Throughout your career, you have been at the forefront of groundbreaking discoveries that have reshaped our understanding of stem cell biology. Your seminal research has unraveled the intricate molecular mechanisms governing stem cell fate determination, providing invaluable insights into the fundamental principles that underlie tissue regeneration and disease pathogenesis. And your relentless pursuit of scientific excellence and unwavering dedication to advancing our understanding of stem cell biology have profoundly impacted both research and clinical practice, inspiring scientists worldwide.
Your pioneering work, which includes the demonstration of stem cell asymmetric division, the proof of the stem cell niche theory, and the discoveries of the Argonaute/Piwi gene family and piRNAs, has illuminated the complex interplay of molecular signals that govern stem cell self-renewal and differentiation. These discoveries have opened, as you have described, a “new world of genes” and new avenues for therapeutic intervention in regenerative medicine.
Beyond your scientific achievements, your visionary leadership and steadfast commitment to mentorship have empowered a new generation of scientists and clinicians, fostering a culture of innovation and collaboration in stem cell research.
Renowned researcher, champion of scientific outreach, and tireless advocate for raising awareness about the transformative potential of stem cell research, you serve as a beacon of inspiration, reaffirming the importance of scientific inquiry in addressing the most pressing challenges facing humanity.
Awarded this 26th day of March, 2024.
Haifan Lin
Haifan Lin is Eugene Higgins Professor of Cell Biology; Professor of Genetics, of Obstetrics, Gynecology & Reproductive Sciences, and of Dermatology at Yale School of Medicine; and Director of the Yale Stem Cell Center. He was elected to the American Academy in 2018.
Thank you, President Oxtoby, for coming all the way to New Haven to bestow this special honor on me on behalf of the American Academy of Arts and Sciences. Thank you also for your many kind remarks. I wish my mother was here to hear these remarks. I also want to thank President Salovey and Dean Brown for hosting this ceremony and celebration. And also for your extremely kind assessment of my work at Yale.
As a faculty member at Yale for over seventeen years, it has been a privilege for me to contribute to this great institution in my own small way. And such contributions are only possible by working closely with many wonderful friends and colleagues, both here at Yale and outside Yale. Having this ceremony in my home institution with dear colleagues and lab members present means so much to me.
I am extremely honored by this prize because it represents a seal of approval of my work from the distinguished American Academy of Arts and Sciences, which was established by the founding fathers of this country. I thank the nominators and the selection committee for their confidence in my work. I feel extremely humbled by this award because, as President Oxtoby mentioned, the past recipients are all movers and shakers in biomedical research who have not only changed reproductive biology and medicine but also how we live our lives.
On this auspicious occasion, I am reminded of an old Chinese saying: “When you drink the water, do not forget those who dug the well.” I am extremely grateful to everyone who has been part of my journey. First of all, I would like to share this honor with my former and current lab members. It is a truly exciting and rewarding experience to work with these brilliant and dedicated young scientists. I also feel extremely blessed to have so many colleagues and friends both at Yale and outside Yale. All of you have always been there for me, and I am grateful to have such kind support and friendship.
I am very fortunate to have a wonderful family, including my amazing goddaughter Christina and her husband Nathan, who are here today. I especially want to acknowledge my wife Edna, who supported my pursuits over many years. She even bought into my theory that spending quality time together is much better than spending a lot of time together.
I would like to take this opportunity to tell you a little bit about my life, about my journey to become a scientist, and about my research. Some forty years ago, when I was a high school graduate, I never imagined I would be here. I graduated from the only high school on a small island in China, off the coast of mainland China, with a population of about forty thousand people.
As a high school student, I was very interested in engineering, but not so much in biology. My engineering aspiration was to become an electrician. Around the time of my graduation, however, genetic engineering had become a very trendy term in China. Lured by the word engineering, I became a biochemistry major at Fudan University in Shanghai. But soon after I entered Fudan, I realized that biochemistry is no engineering—yet it is even more intriguing!
To broaden my training in biology, after I graduated from Fudan, I went to Cornell to pursue my PhD training in developmental biology and genetics. Consequently, China lost a potentially talented electrician!
While at Cornell I worked on early embryogenesis guided by Professor Mariana Wolfner. During that period of training, I became interested in many questions in developmental biology. By the time I graduated I was particularly interested in stem cells.
At that time, stem cells were an esoteric field, so I needed to find a lab that could accommodate my interest. I was very lucky to find Dr. Allan Spradling’s lab at Carnegie Institution of Washington, and he took me in. In his lab I started to work on stem cells using fruit fly ovaries as a model. I took this project to Duke, where I started my own lab, and there I also expanded stem cell research to the mammalian side.
I had twelve incredible years at Duke, and I was thinking I would spend the rest of my career in North Carolina and then retreat to Florida to enjoy my geriatric spring break. However, in 2006 my life took an unexpected turn when Yale called to ask if I would be interested in being a candidate to establish the Yale Stem Cell Center. A call from Yale? I better take it seriously. Once I visited the campus, I fell in love with this place, and I have been here ever since. I also fell in love with New Haven, so much so that I gave up the Florida idea!
The Academy asked me to share some of my research with you. For those of you who are familiar with my work, now is a good time to take a nap.
I work on mechanisms that define stem cell properties. As you may know, metaphorically, stem cells are the mother of all cells. So then what are stem cells? There are two types of stem cells: 1) embryonic stem cells that exist in early embryos; and 2) adult tissue stem cells that exist in most of our adult tissues.
Embryonic stem cells give rise to all cells in our bodies, such as pancreatic cells, bone, and neurons. However, adult stem cells can normally only give rise to cells in their resident tissues. For example, neural stem cells can only give rise to neurons and accessory cells in the nervous system, but not to any cells outside the nervous system.
Despite the big differences between these two classes of stem cells, they share a very important ability, namely the ability to renew themselves. Because of this ability, stem cells become the fountain of youth in their resident tissues. This ability fascinated me and became my career-long pursuit.
When I entered the stem cell field, there were two important hypotheses that became the two pillars of stem cell biology. One is the asymmetric division hypothesis, which was first proposed 140 years ago by German biologist Theodor Boveri. He proposed that when a stem cell divides, it will generate a daughter stem cell identical to itself and another daughter cell that is committed to more specific functions (so-called differentiated cells).
When the daughter stem cell further divides, it will generate another daughter stem cell and another differentiated cell. So when the original stem cell divides three times, it will still maintain a copy of itself, while generating three differentiated cells. This renders the stem cell’s ability to self-renew. This asymmetric division became an earlier part of my research interest.
The second important hypothesis in the stem cell field, called the stem cell niche theory, was first proposed by British scientist Ray Schofield in 1978 when he observed that blood stem cells are not as well supported by the spleen as by bone marrow. This hypothesis says that stem cells normally reside in an idyllic hideaway in a tissue that is composed of niche cells and their effective niche signaling field. When a stem cell is in this paradise, or Shangri-la if you wish, it self-renews and lives forever. But once a stem cell leaves this paradise, it is doomed to differentiate and die young.
Although these two hypotheses are important and elegant, they had not been proven when I started my work because of two unique challenges then in stem cell research—and to a large degree, these challenges still exist today. The first challenge is what I call identity crisis. Stem cells look no different from their neighboring cells, and they exist in extremely small numbers. Therefore researchers often don’t know if what they are working on are true stem cells. This precludes any in-depth analysis of stem cells. The second challenge is that stem cells are the biological equivalent of quantum particles that obey Heisenberg’s Uncertainty Principle. Namely, when you touch them, they will change their property. This also precludes any in-depth type of mechanistic studies.
To overcome these two problems, I decided to work on stem cells in little fruit flies because this little organism has been a powerful genetic model for nearly a century, and studying them has led to the discovery of many important medical mechanisms that apply to humans.
I focused on the fruit fly ovary because it contains strings of developing egg chambers, and they are all produced from the apical tiny tips of the strings. It had been proposed that egg-producing stem cells reside in these apical tips. However, they have not been directly identified. Given this situation, I decided to study stem cells using the following strategy: First, I wanted to know if stem cells indeed exist in the fruit fly ovary. If so, where? Then, I wanted to know if these stem cells divide asymmetrically. After that, I wanted to know if there is a niche for stem cells. Finally, I wanted to identify genes involved in regulating stem cell function.
With this strategy, I started to address the question of whether stem cells exist in the fruit fly ovary. I devised a method that allows me to isolate that tiny ovarian tip and transplant it into a recipient female whose ovary has been removed. After the transplantation, a complete ovarian unit was generated within three days: In twelve hours, it had created new egg chambers. Another twelve hours and there were new developing eggs. And by day three it had generated the entire ovary.
This experiment shows that the tiny tip does indeed contain stem cells. So the next question is, where are these stem cells in the tiny tips? I used laser ablation to kill individual germ cells. And I found that when I killed the two anterior germ cells, the entire egg production process stopped. But when I killed any germ cells next to it, it did not even slow down the rate of egg production. This analysis allowed us to know that these two cells are the egg-producing stem cells, and other cells are the differentiated cells. In the process, I also found the cap cells, which hug the stem cells.
Identification of stem cells in a Tissue by Laser Ablation
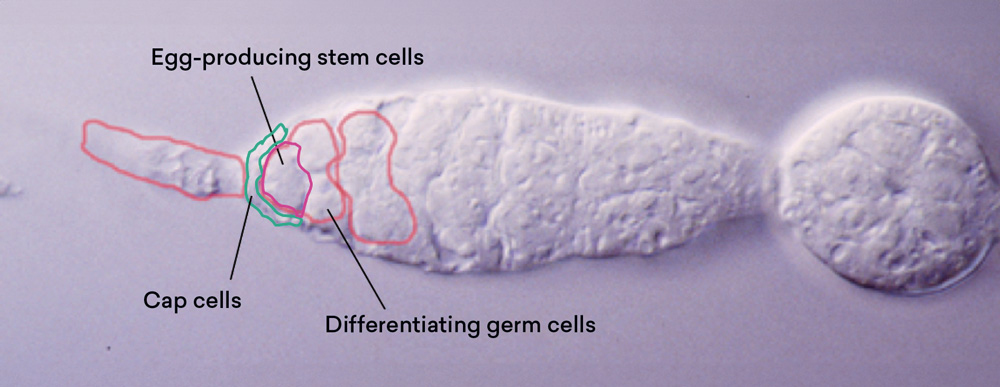
Source: Haifan Lin and Allan C. Spradling, “Germline Stem Cell Division and Egg Chamber Development in Transplanted Drosophila Germaria,” Developmental Biology 159 (1) (1993): 140–152.
Germline stem cells indeed divide asymmetrically!
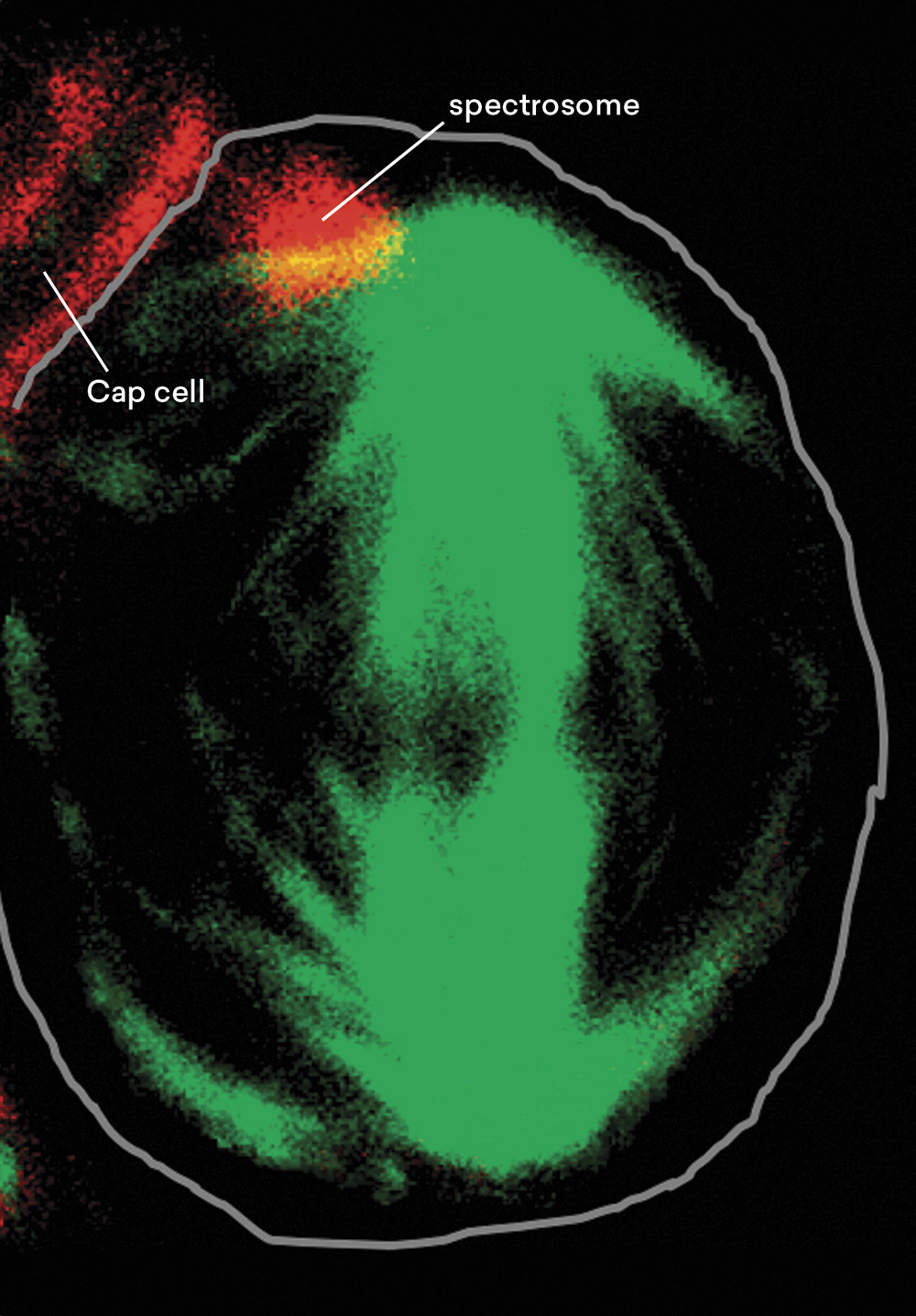
Source: Haifan Lin and Allan C. Spradling, “A Novel Group of Pumilio Mutations Affects the Asymmetric Division of Germline Stem Cells in the Drosophila Ovary,” Development 124 (12) (1997): 2463–2476; and Wei Deng and Haifan Lin, “Spectrosomes and Fusomes Anchor Mitotic Spindles during Asymmetric Germ Cell Divisions and Facilitate the Formation of a Polarized Microtubule Array for Oocyte Specification in Drosophila,” Developmental Biology 189 (1) (1997): 79–94, cover story.
Now that we know where the stem cells in the ovary are located, the next question becomes do germline stem cells divide asymmetrically as proposed 140 years ago? The image above shows a stem cell in division caught in action. Biologists in the room know that this green structure inside the stem cell is called the mitotic spindle. It is a structure required for cell division, and the two poles of the structure will define the position of the two daughter cells.
As we can see, after the stem cell divides, one daughter cell will still remain in contact with the cap cell shown in red and will still be a stem cell. Whereas the other daughter cell will be one cell away from the cap cell and will become a differentiated daughter cell.
So, we have a clear asymmetry in stem cell division. In the process, we also discovered a new subcellular structure that we named the spectrosome, which can anchor one pole of the mitotic spindle. The spectrosome not only marks but also helps establish the asymmetry of the division.
Now that we know where the stem cells are and how they divide, we next want to know if a niche exists for these egg-producing stem cells. We found that when we ablated these anterior somatic cells (namely, ordinary body cells), this partial ablation affected stem cell behavior. This initial observation and subsequent molecular analysis tell us that these cells are actually niche cells for the stem cells.
Asymmetric Stem Cell Division within a Niche
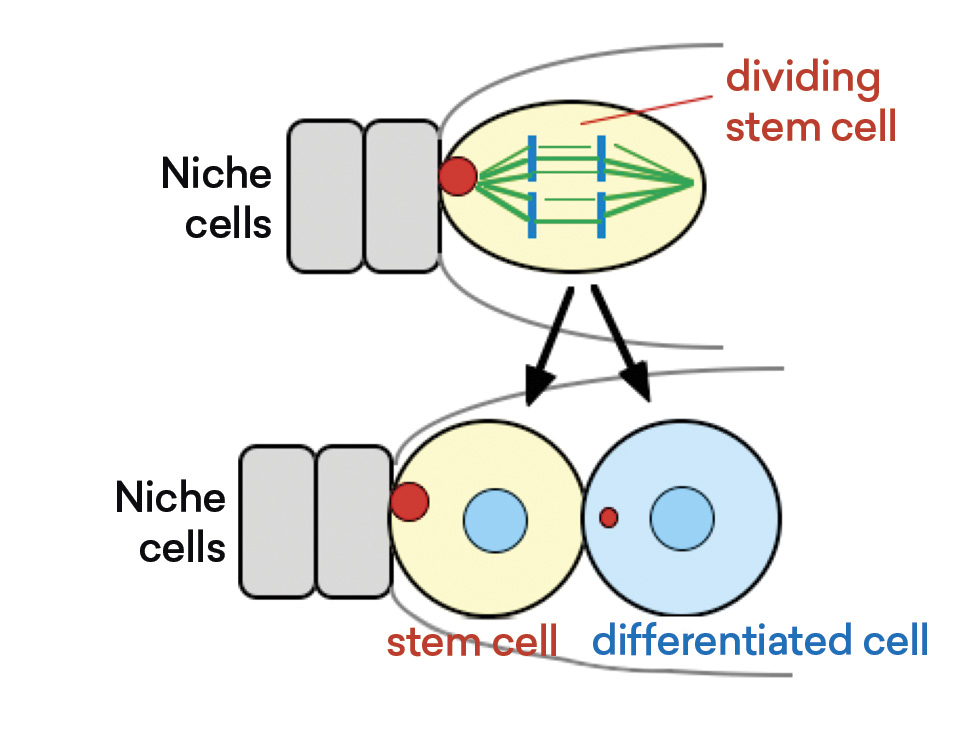
Source: Wei Deng and Haifan Lin, “Spectrosomes and Fusomes Anchor Mitotic Spindles during Asymmetric Germ Cell Divisions and Facilitate the Formation of a Polarized Microtubule Array for Oocyte Specification in Drosophila,” Developmental Biology 189 (1) (1997): 79–94, cover story.
Let me summarize what we have found so far. By using a simple organism as a model, we were able to unambiguously identify stem cells and their niche cells and show the asymmetric division of stem cells and the niche function in this process.
So now the question is, what genes control the asymmetric division and niche function of stem cells?
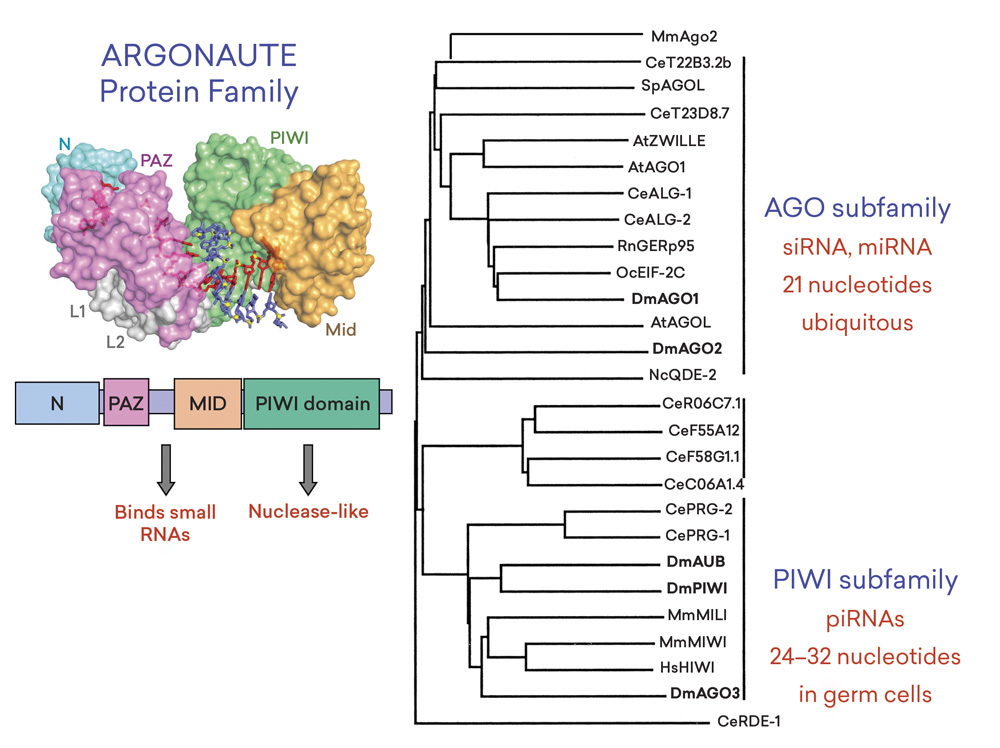
Daniel N. Cox, Anna Chao, Jeff Baker, Lisa Chang, Dan Qiao, and Haifan Lin, “A Novel Class of Evolutionarily Conserved Genes Defined by piwi are Essential for Stem Cell Self-Renewal,” Genes & Development 12 (23) (1998): 3715–3727. 3D structure by Yanli Wang et al., “Structure of an Argonaute Silencing Complex with a Seed-Containing Guide DNA and Target RNA Duplex,” Nature 456 (2008): 921–926.
As Peter, David, and Nancy alluded to in their remarks, we discovered a number of very important genes. And using a particular gene family, the Argonaute/Piwi gene family, as an example, we found that it encodes proteins that have four localized structures. Leemor Joshua-Tor, Dinshaw Patel, and other researchers resolved these structures at near atomic resolution. These proteins can bind to small RNAs and sometimes can also cleave RNAs. This gene family can be divided into Argonaute and Piwi subfamilies. The Argonaute subfamily of proteins binds to small interfering RNAs and microRNAs, and they play a very important role in regulating gene expression in most cell types.
Andy Fire (Stanford University) and Craig Mello (University of Massachusetts Chan Medical School), both members of the American Academy, discovered the RNA interference mechanism. That discovery had a revolutionary impact not only on research but also on patient treatment. My lab has been focused on the Piwi subfamily gene because they are mostly expressed in the germline and primitive stem cells. In 2006, my lab and three other labs independently discovered another class of small non-coding RNAs—meaning they don’t make proteins—that interact with Piwi proteins. We call them Piwi-interacting RNAs, or piRNAs for short. These piRNAs are mostly 24–32 nucleotides long. They bind to Piwi proteins, and they also exist only in the germline, i.e., reproductive cells, and primitive stem cells.
One amazing thing about these piRNAs is that they are encoded by genes that are hiding in the part of the genome that people used to call “junk” DNA. Many of you may know that the study of molecular biology over the past several decades has been focused on the central dogma, which tells us that genetic information in DNA is transcribed into mRNAs, which then instruct the production of proteins, and that is how life gets started. The discovery of miRNAs and siRNAs further enriched this dogma.
However, this dogma only pertains to about 1 percent of our human genome. What about the other 99 percent of that genome, the junk DNA? I prefer the term “terra incognita” because we know so little about these junk DNA.
To our surprise, we found that these piRNA-encoding genes are actually hiding in these junk DNA regions. Today, my lab has discovered more than 16 million piRNAs even in a simple organism like hydra. This number is at least seven hundred times larger than the total number of known genes in our genome. We were euphoric about this discovery. If you liken the genome to the world, then the traditional protein-coding genes are like the Old World. Suddenly we found ourselves landed ashore in a completely New World with a new type of genes and a new type of gene products. In 2006, Science magazine rated this discovery as one of the ten breakthroughs that year.
Hoopla aside, a very important question still remains. Are these piRNAs functional in any way? For the past ten to fifteen years we have been working on that question. Our recent work indicates that Piwi proteins and piRNAs together represent a new paradigm of whole-genome regulation in reproductive cells.
Our genome is very complex. It contains about 23,000 traditional genes. In addition, our genome also contains about 1 million copies of jumping genes called transposons, which are viewed as the parasites of the genome. Moreover, my lab and others found the piRNA-coding genes. In addition, a number of other labs discovered yet another type of genes, namely genes that make tens of thousands of long RNAs that do not encode protein information, called long noncoding RNAs, or lncRNAs for short. Furthermore, our genome also contains more than 30,000 pseudogenes that look like real genes, but they are nonfunctional and are often viewed as the dead carcass of real genes on their way out during evolution. Lastly, our genome also contains centromeres that are important for chromosome segregation, and telomeres that protect our genome from being shortened.
Our recent studies showed that piRNAs actively regulate gene expression, and that piRNAs also regulate transposon activity and lncRNA expression. In addition, transposons can regulate traditional gene expression. Pseudogenes also actively regulate traditional gene expression, both through the piRNA pathway. Finally, we showed that Piwi and piRNA can also regulate the function of centromeres and telomeres. Thus, the Piwi-piRNA pathway represents a new paradigm that unites the entire genome. This uniting function is indeed much needed in today’s real world.
In the past few years, we have found that this new paradigm of gene regulation has important medical implications. For example, when they become overly active, they promote breast cancer, gastric cancer, seminomas, prostate cancer, liver cancer, and colorectal cancer. We are excited about the possibility of developing new cancer-treatment strategies based on these new mechanisms.
Reflecting on my research, I have been very fortunate to have had the opportunity to work closely with generations of students and postdoctoral fellows at Duke and at Yale. I want to thank all of you for your friendship, for your support, and for being here today with me on this special occasion.
© 2024 by Peter Salovey, Nancy J. Brown, and Haifan Lin, respectively
To view or listen to the presentation, please visit the Academy’s website.




